That booty
means business
Butt First, change the way you treat cellulite with Qwo®
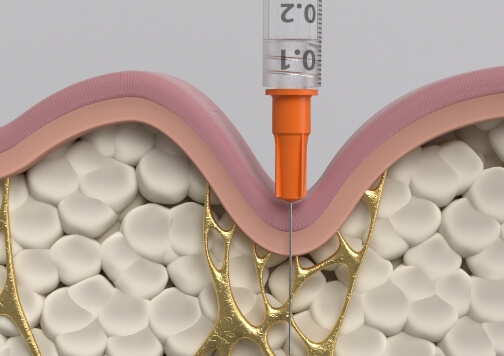
QWO is the first and only FDA-approved injectable treatment for moderate to severe cellulite in the buttocks of adult women.

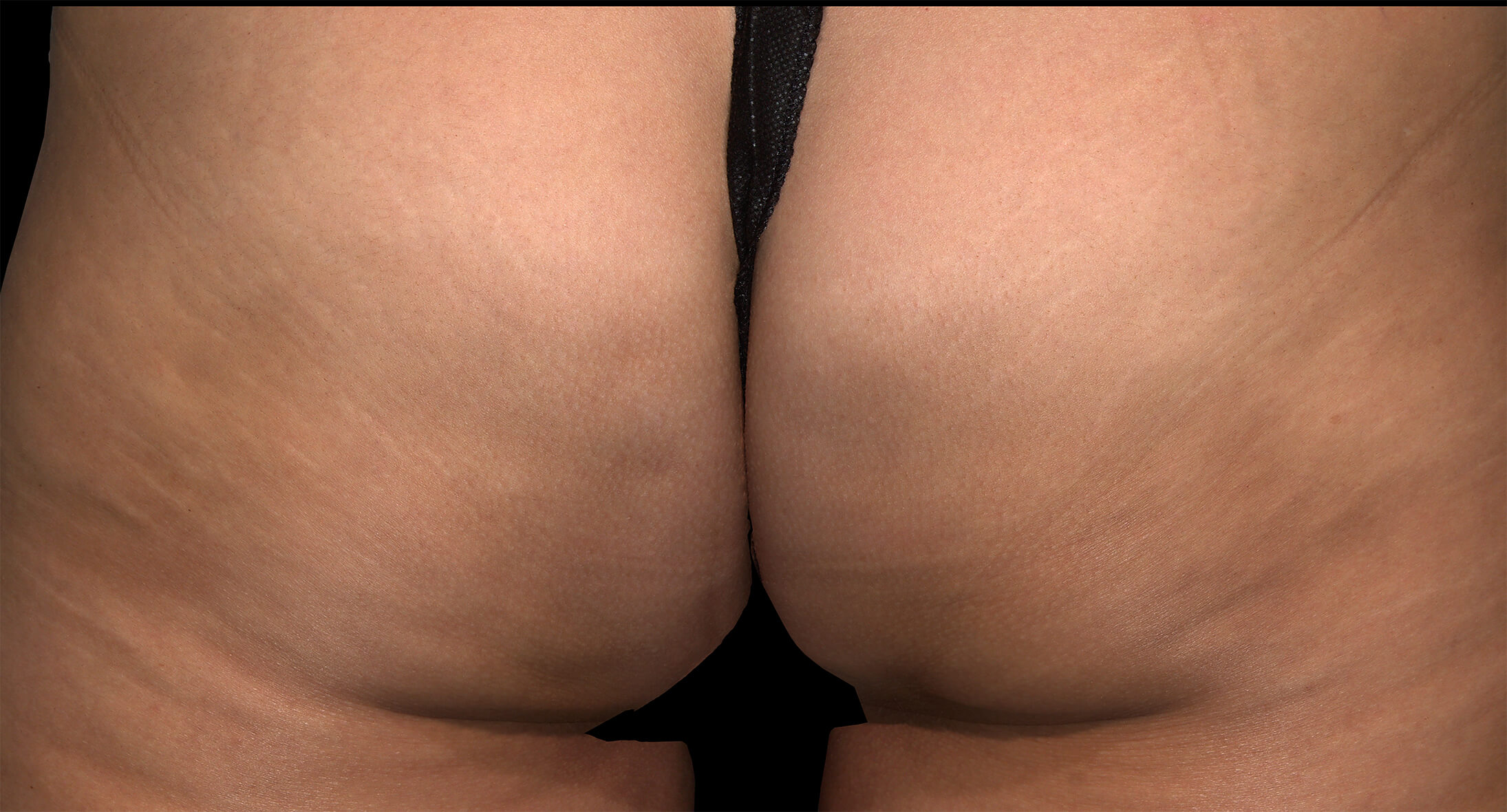
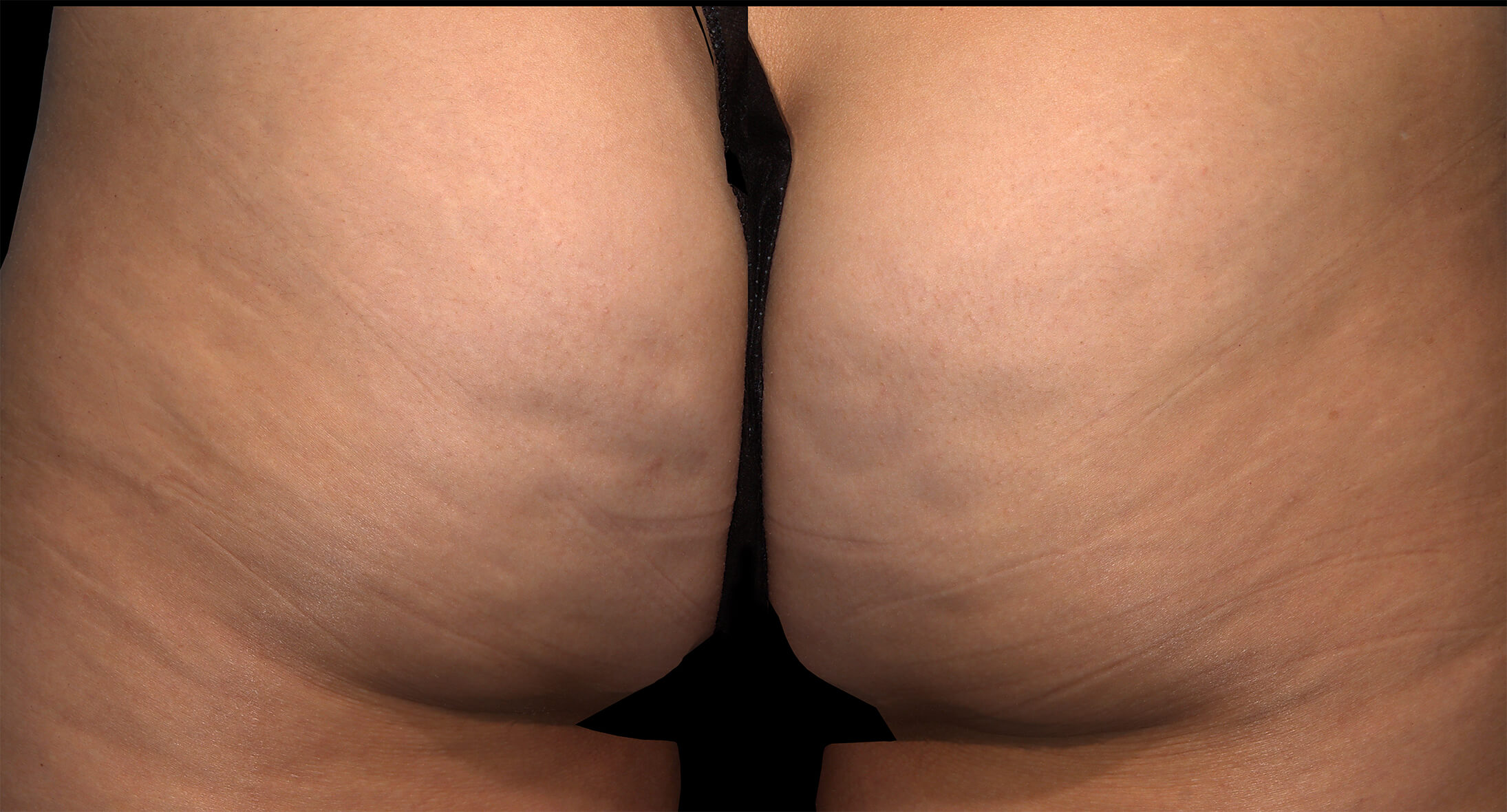
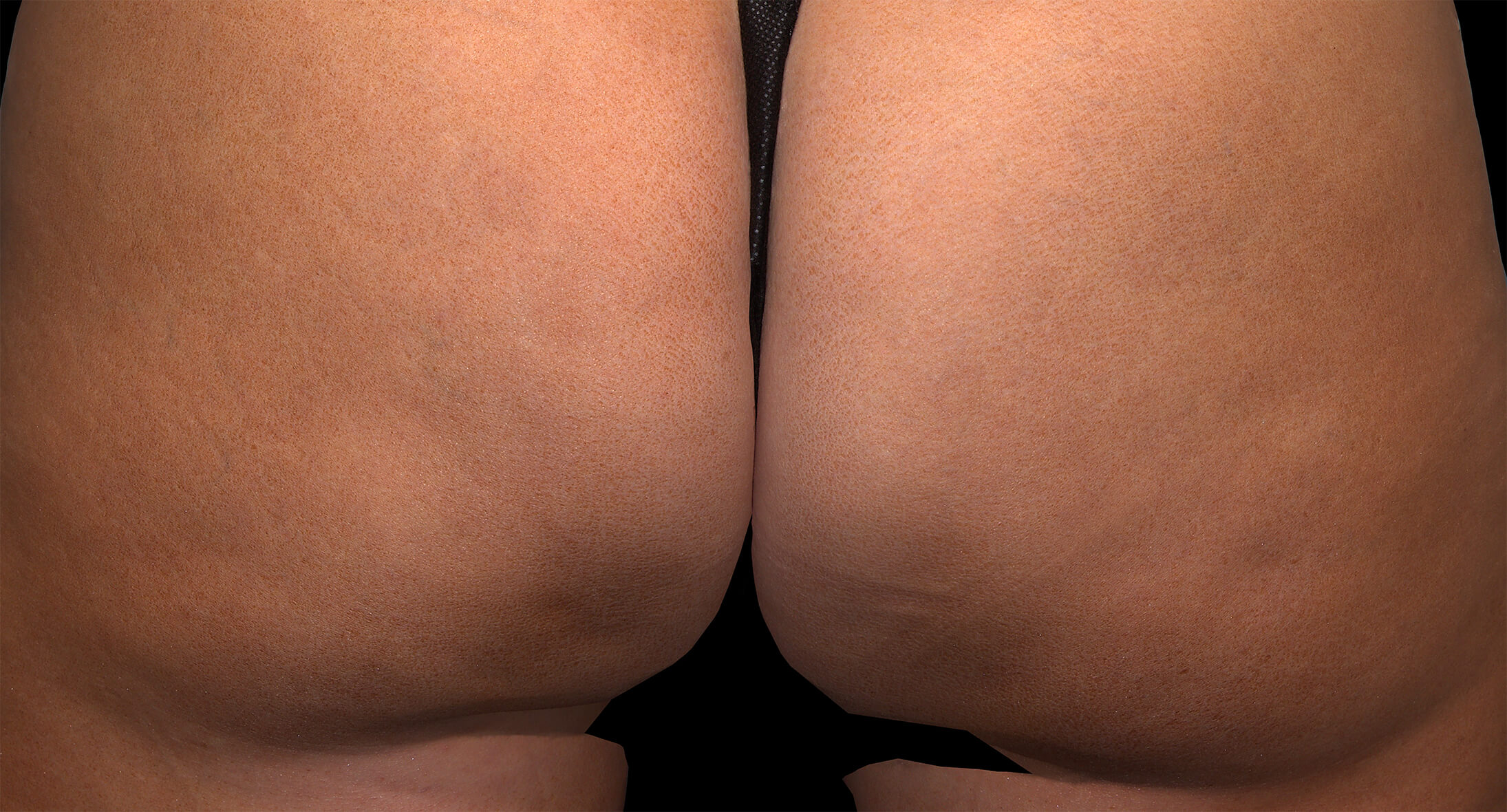
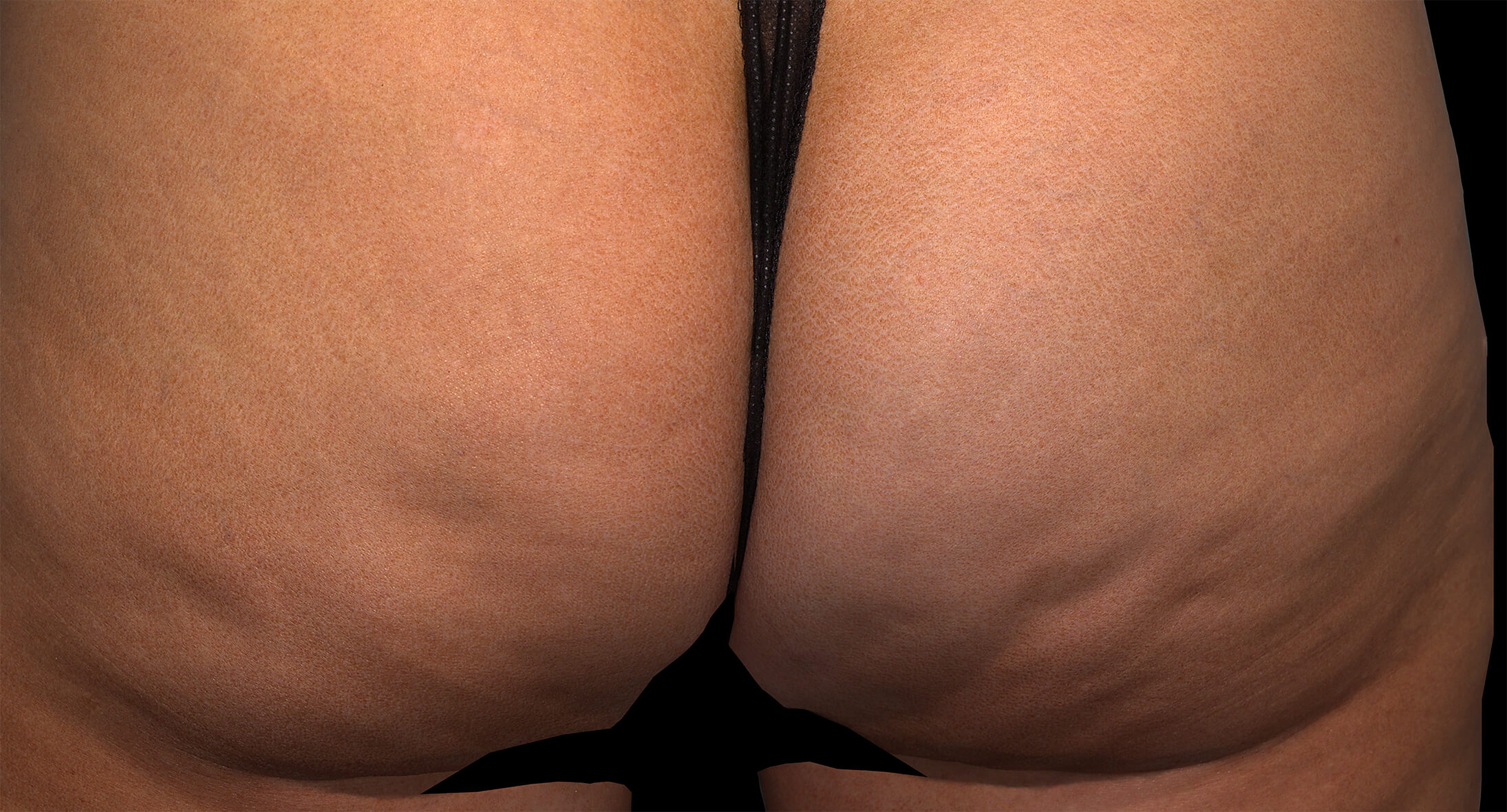
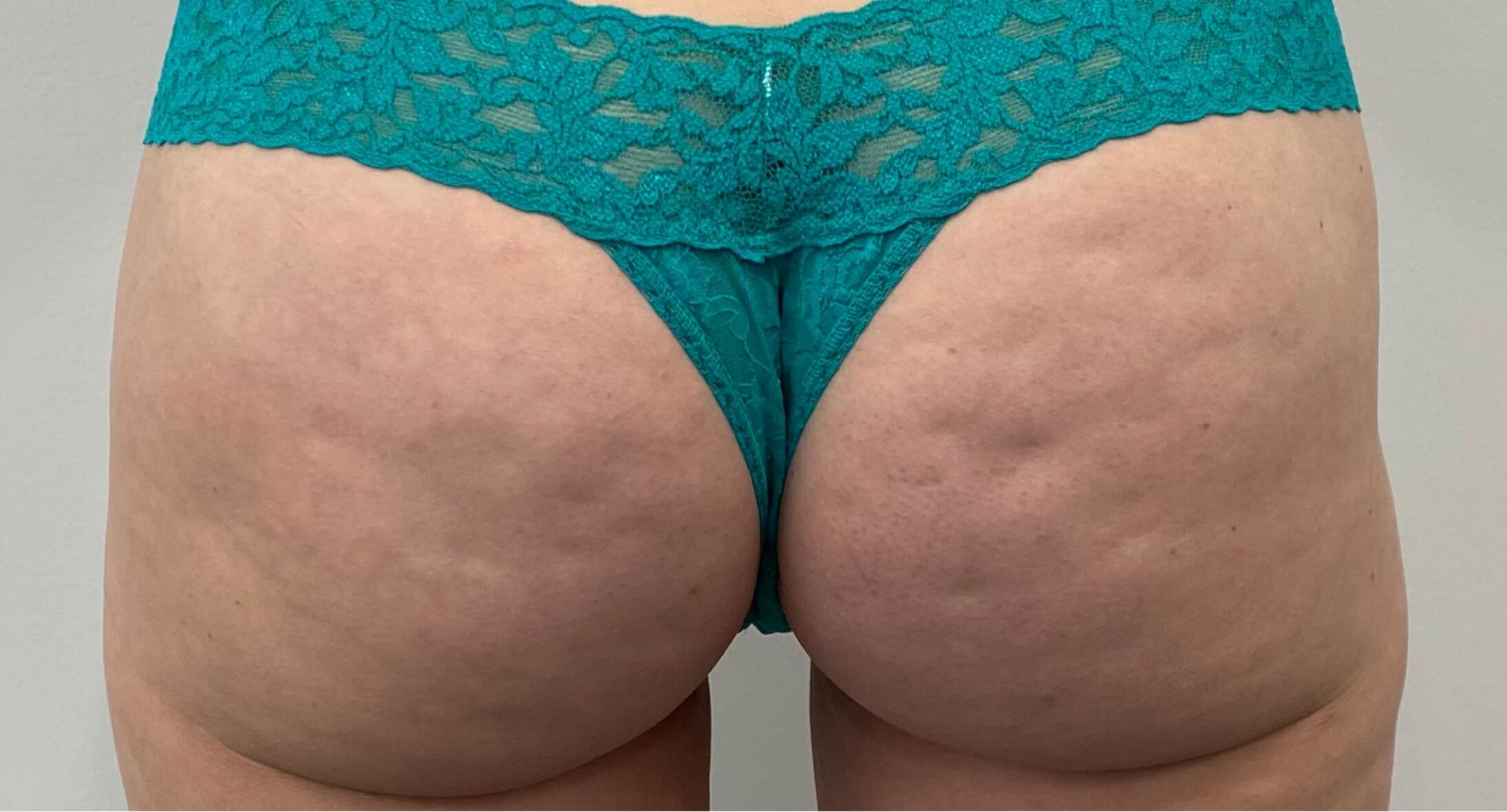
Before & after results
The Opportunity for
Your Practice
The QWO difference:
Studied in more than 1,800 patients, QWO is the most clinically studied FDA-approved treatment for cellulite available.2
Visible results 28 days after the third treatment (21 days apart).1,3*
*In clinical trials, efficacy was assessed at Day 71.
Individual results may vary.
In the clinical trials, no post-treatment downtime was required.4
As seen on

“I am thrilled there is now an FDA-approved injectable treatment option for cellulite.”
– Anne Chapas, MD
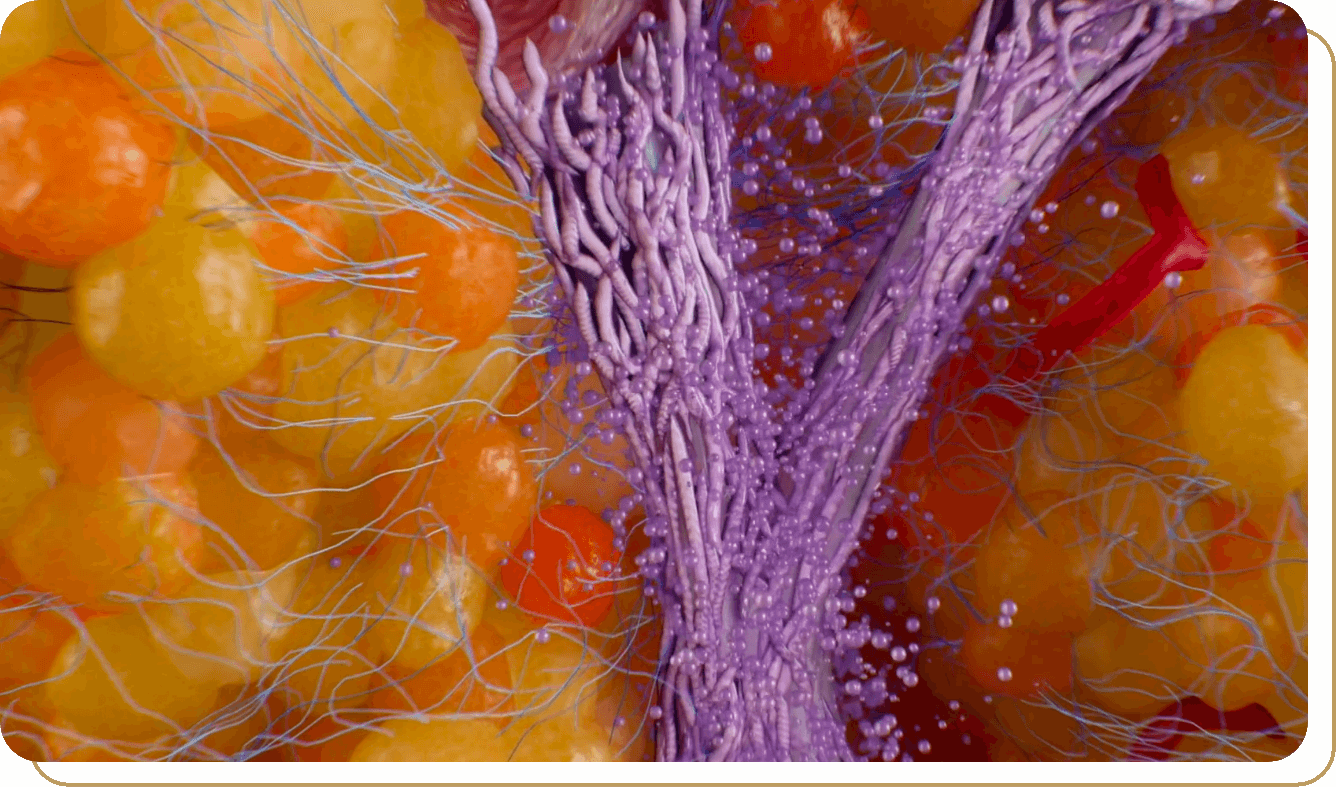
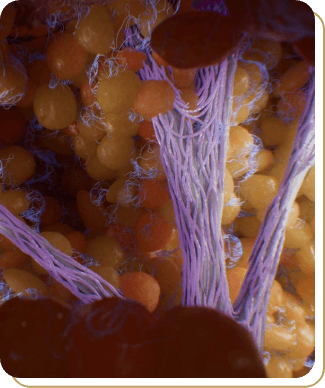
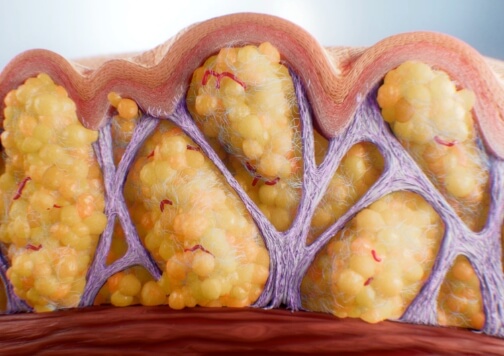
Perceived Mechanism of Action
The exact mechanism for the treatment of moderate to severe cellulite is unknown.3
Learn MoreClinical Education Center
Here you will find additional information and resources.
-

-

-

Publications & presentations
Read up on a variety of publications and presentations on QWO
Learn more -

Want to learn more about QWO?
Head over to the Clinical Education Center for more information.
Mechanism of Action Video