Indication
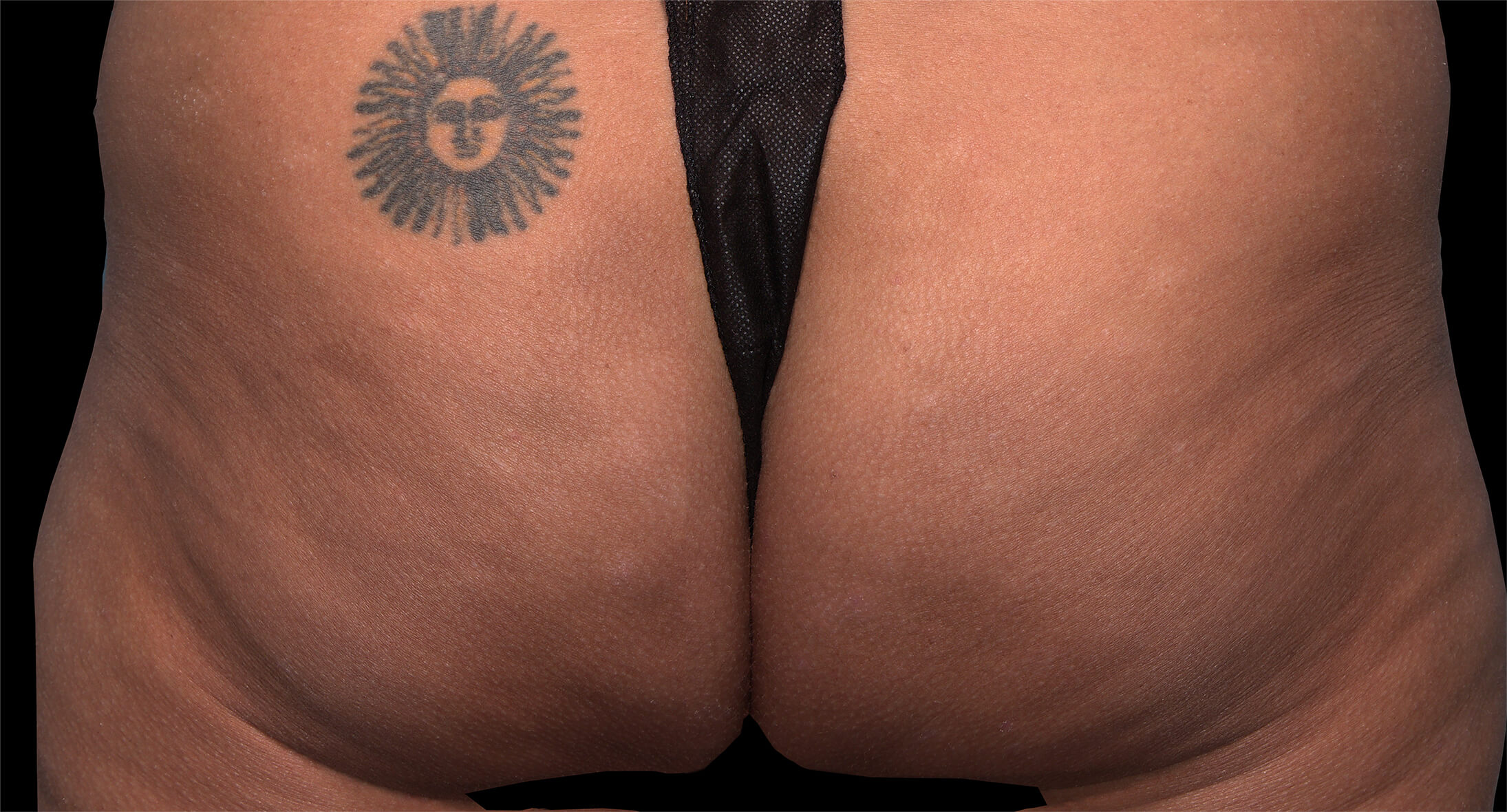
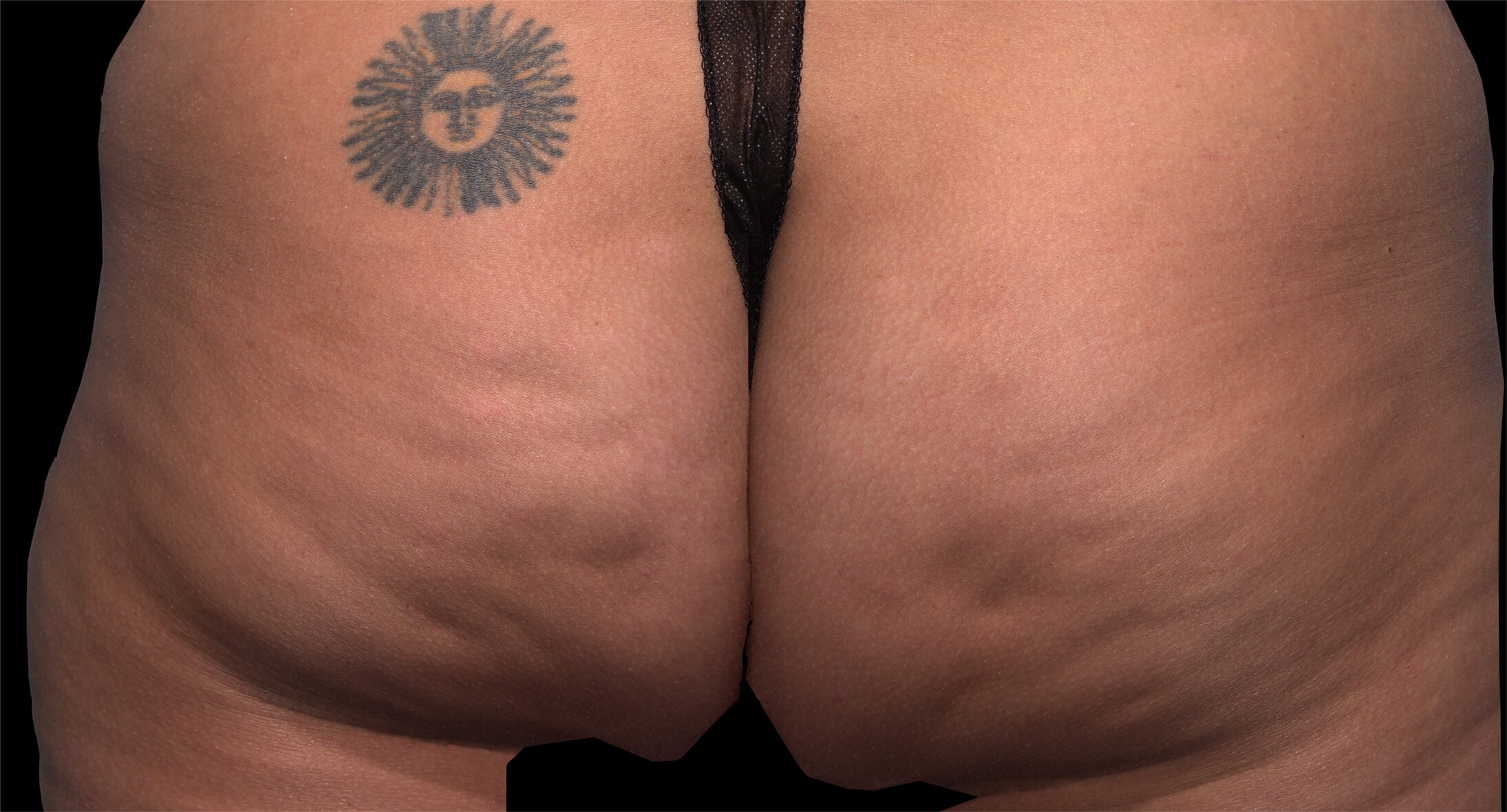
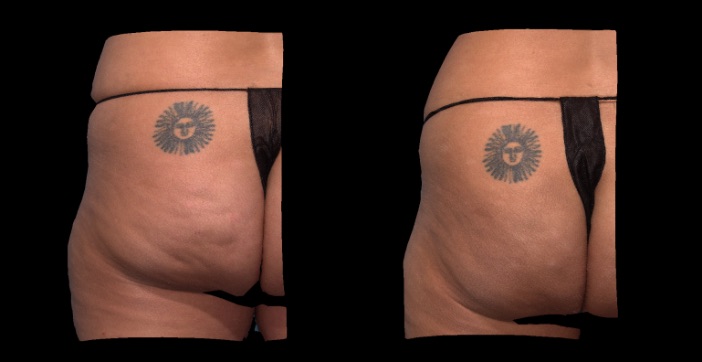
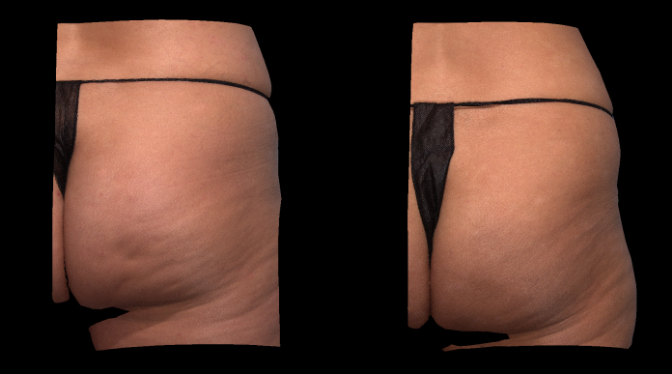
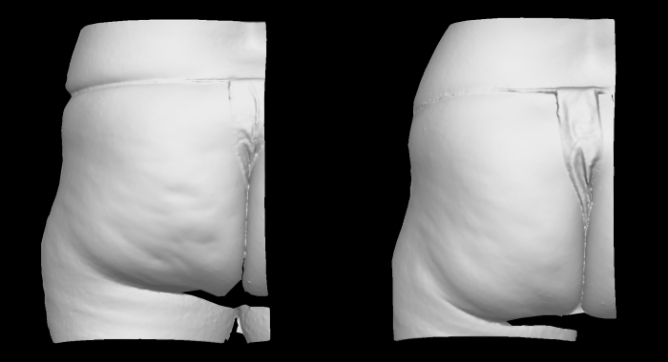
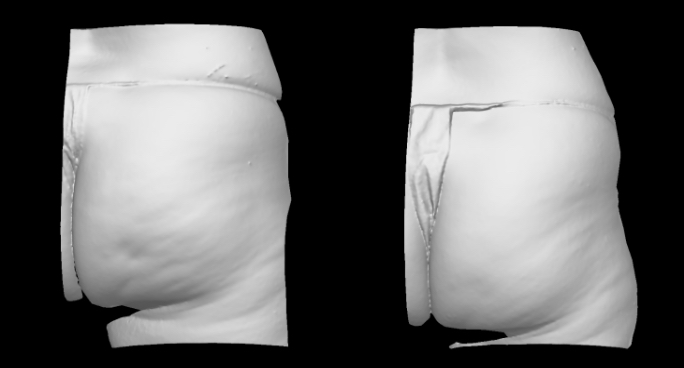
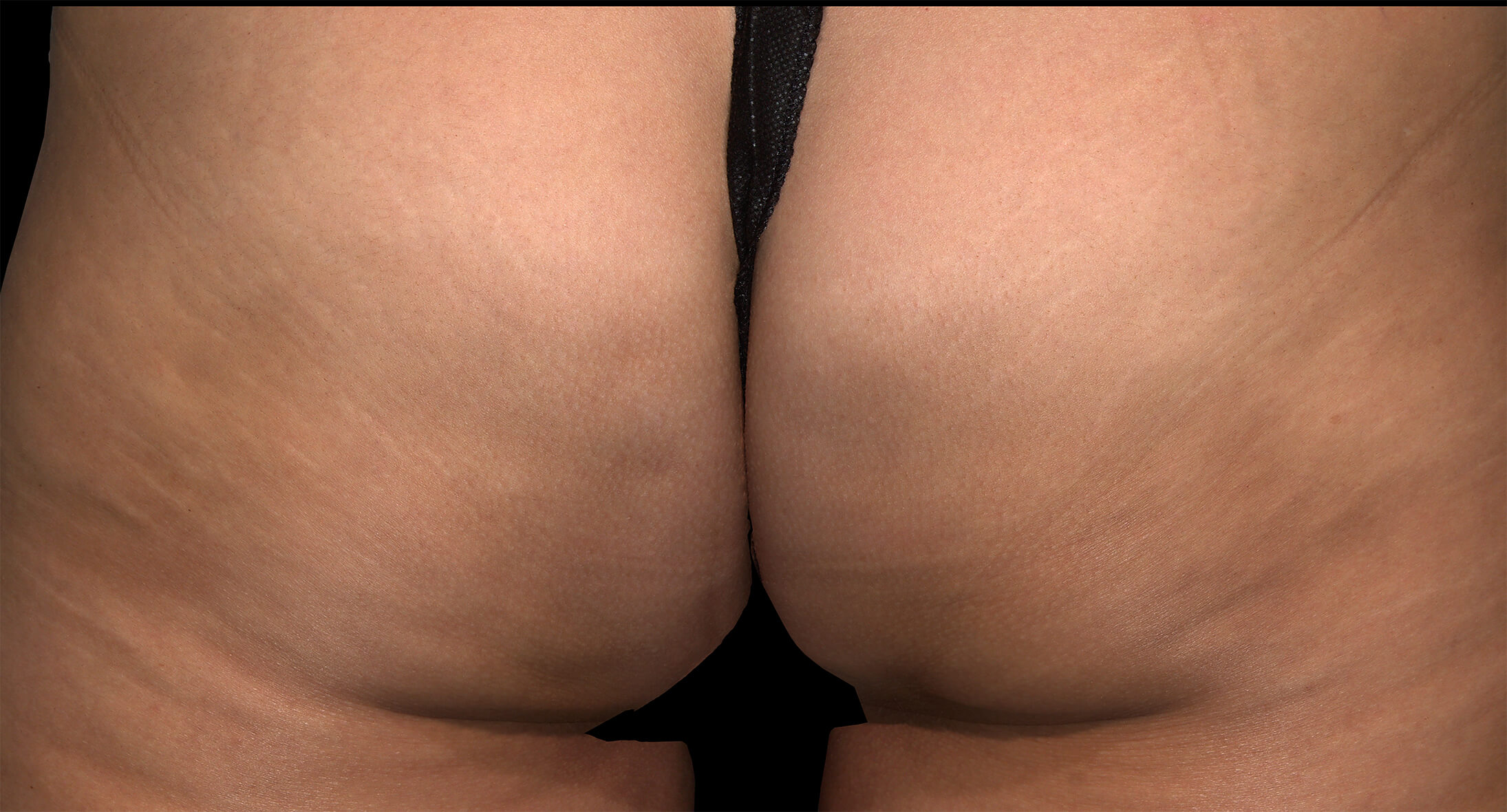
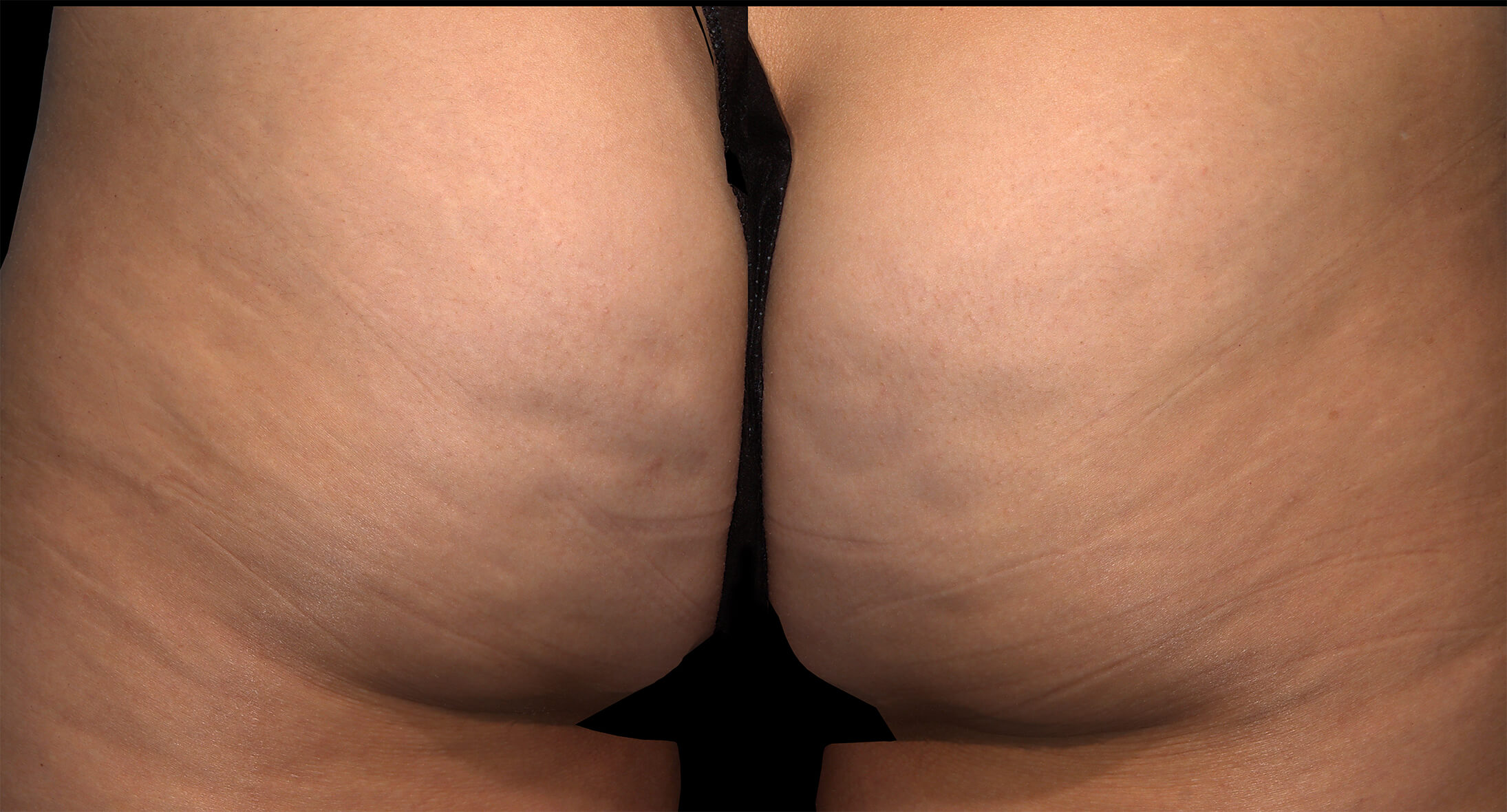
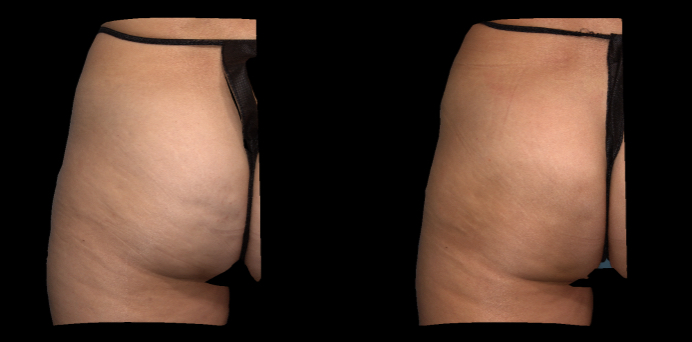
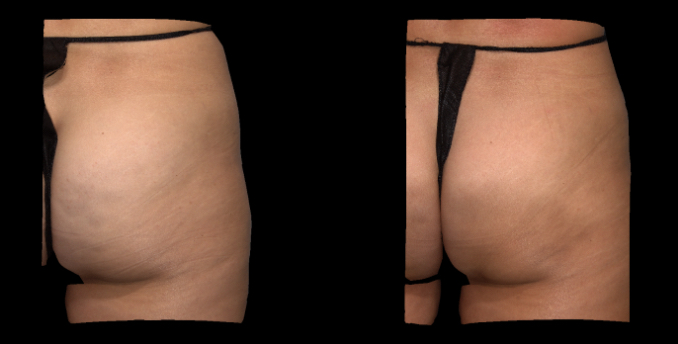
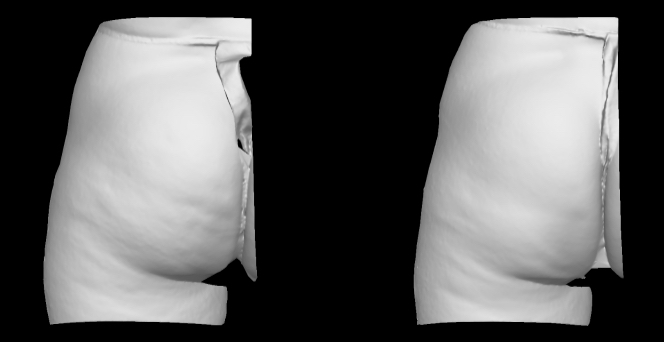
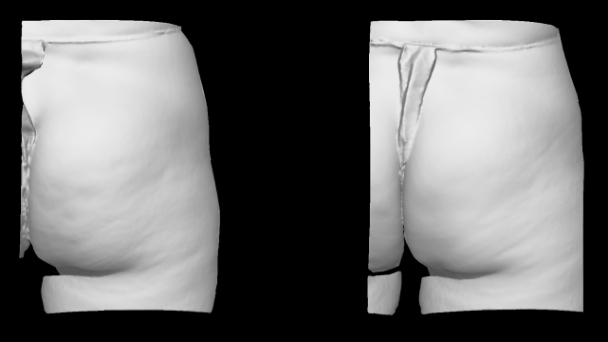
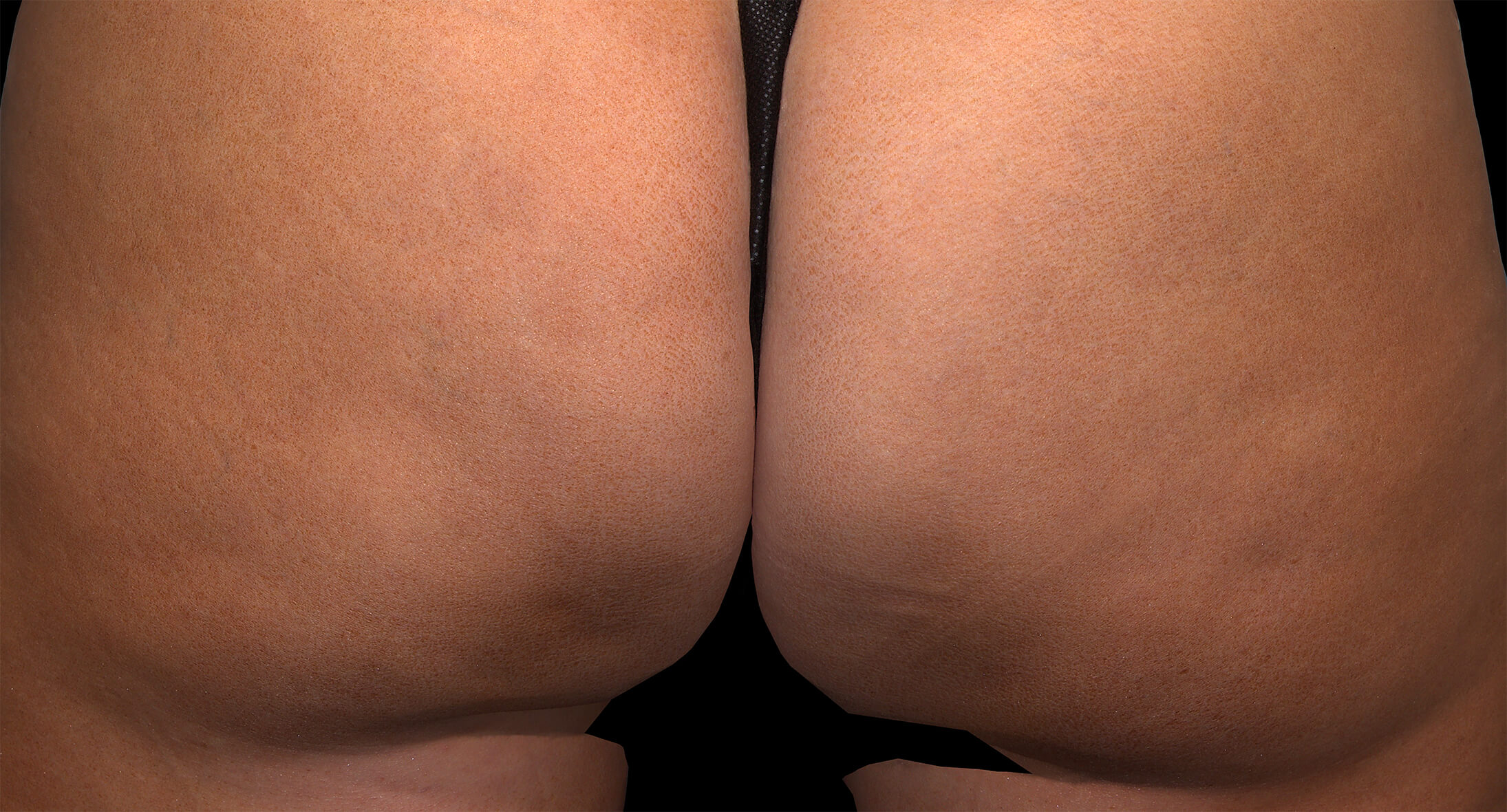
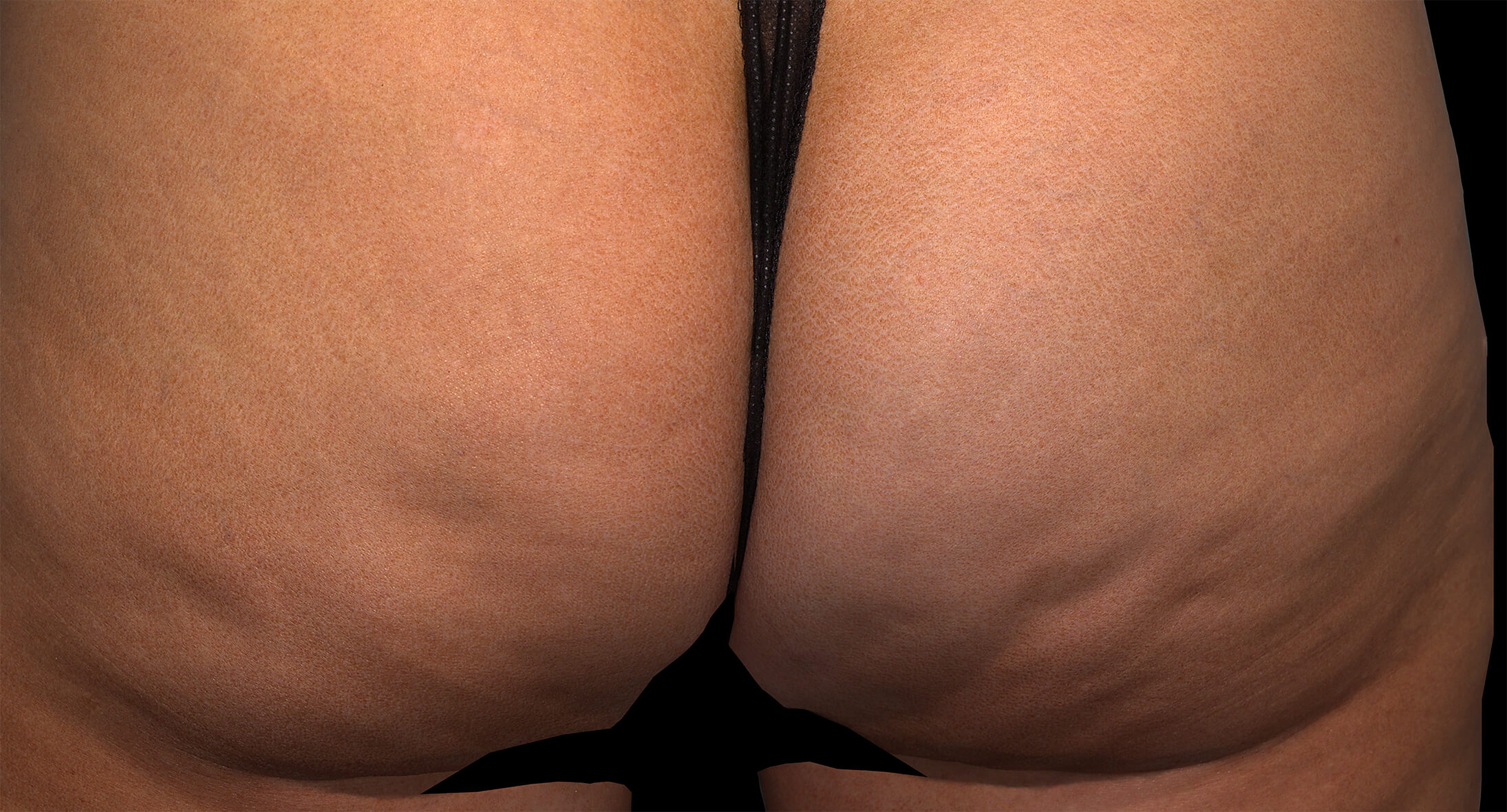
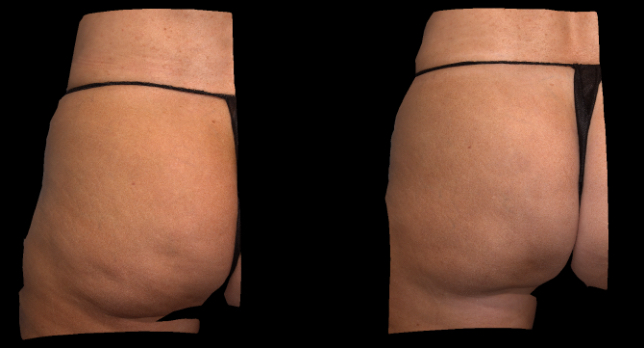
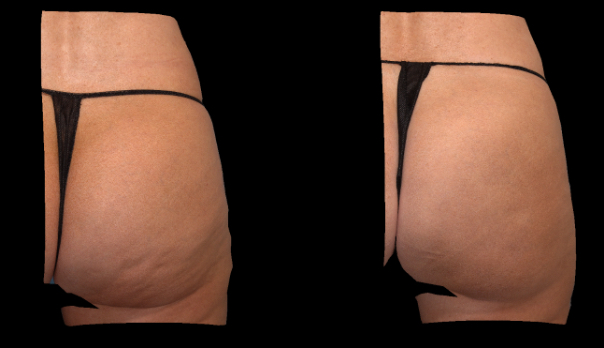
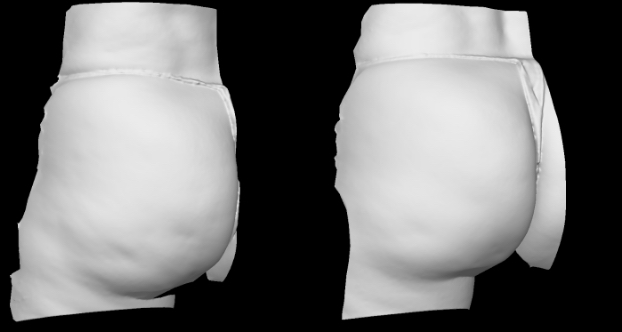
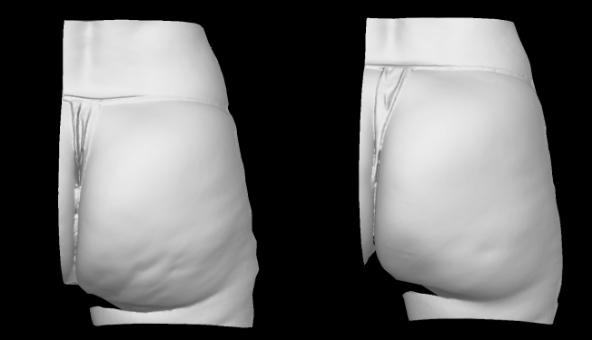
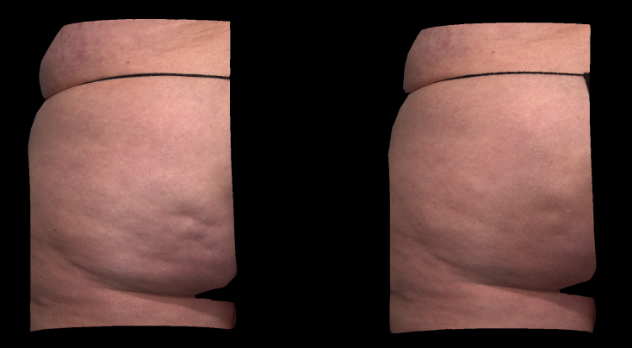
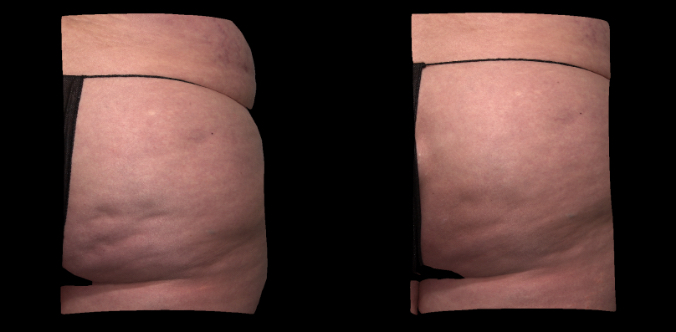
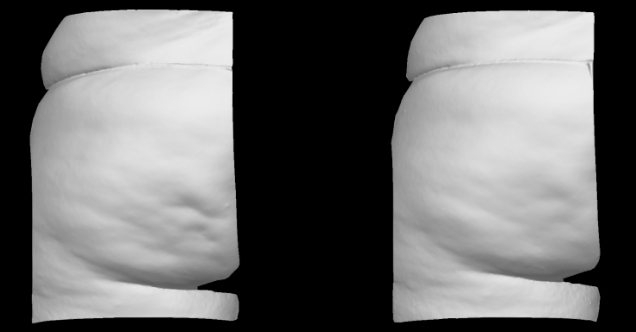
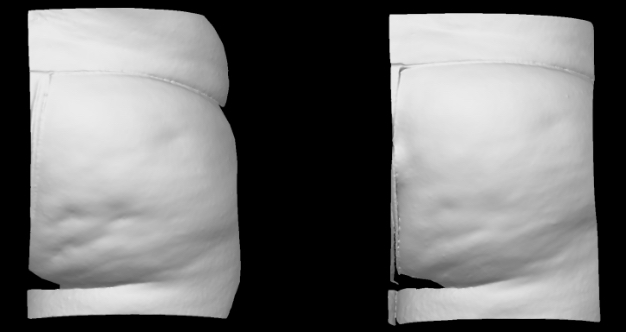
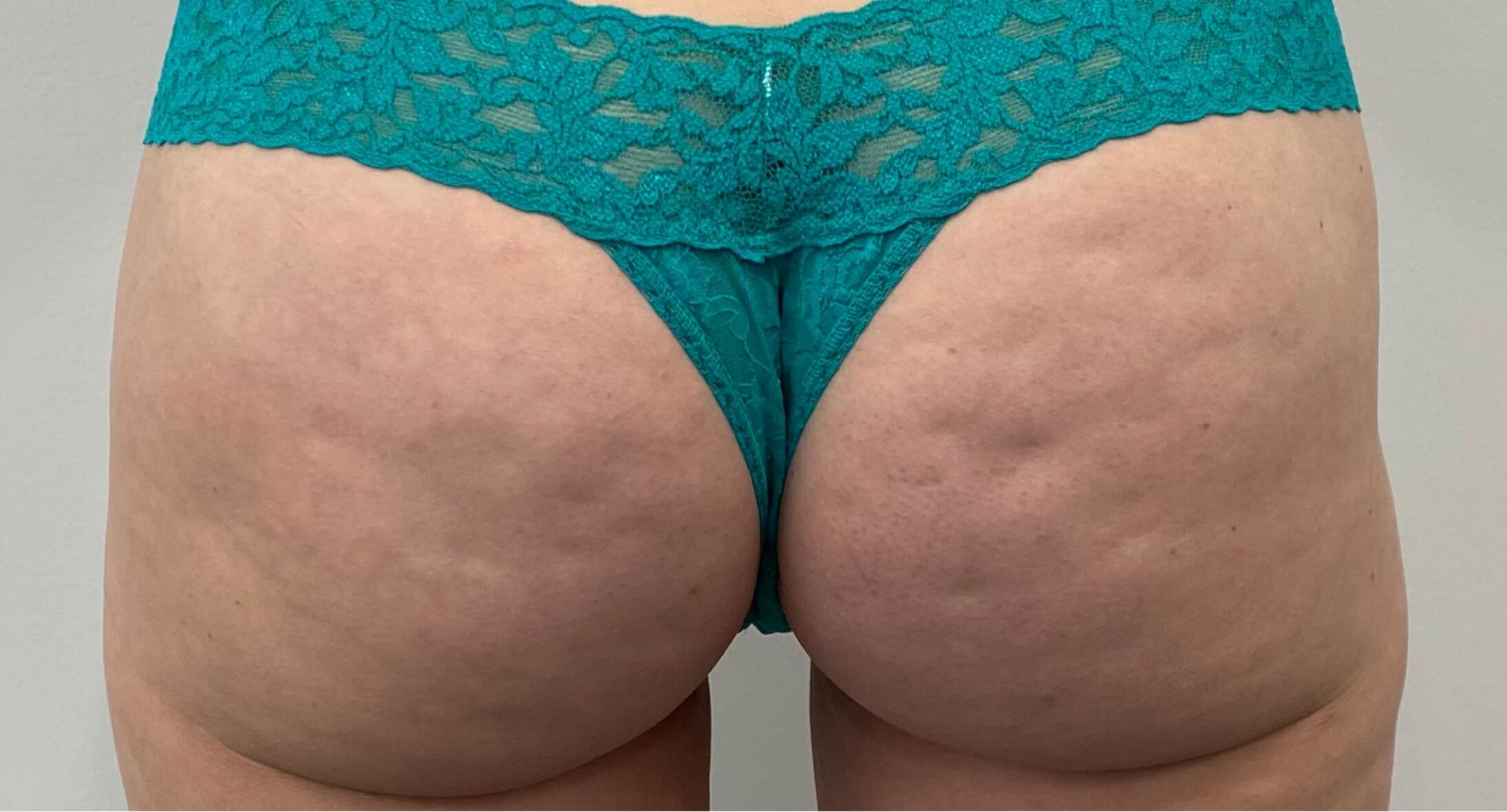
Qwo® is indicated for the treatment of moderate to severe cellulite in the buttocks of adult women.
Important Safety Information for QWO
Contraindications
QWO is contraindicated in patients with a history of hypersensitivity to collagenase or to any of the excipients or the presence of infection at the injection sites.
Warnings and Precautions
Hypersensitivity Reactions
Serious hypersensitivity reactions including anaphylaxis have been reported with the use of collagenase clostridium histolyticum. If such a reaction occurs, further injection of QWO should be discontinued and appropriate medical therapy immediately instituted. Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
Injection Site Bruising
In clinical trials, 84% of subjects treated with QWO experienced injection site bruising. Subjects with coagulation disorders or using anticoagulant or antiplatelet medications (except those taking ≤150 mg aspirin daily) were excluded from participating in Trials 1 and 2.
QWO should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet (except those taking ≤150 mg aspirin daily) or anticoagulant therapy.
Substitution of Collagenase Products
QWO must not be substituted with other injectable collagenase products.
QWO is not intended for the treatment of Peyronie’s Disease or Dupuytren’s Contracture.
Adverse Reactions
In clinical trials, the most commonly reported adverse reactions in patients treated with QWO with an incidence ≥ 10% were at the injection site: bruising, pain, nodule and pruritus.
View Full Prescribing Information for QWO.