Safety
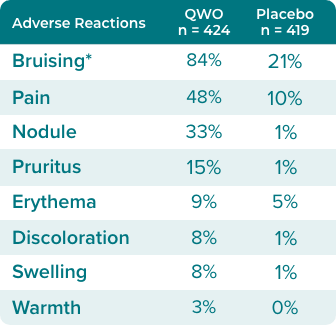
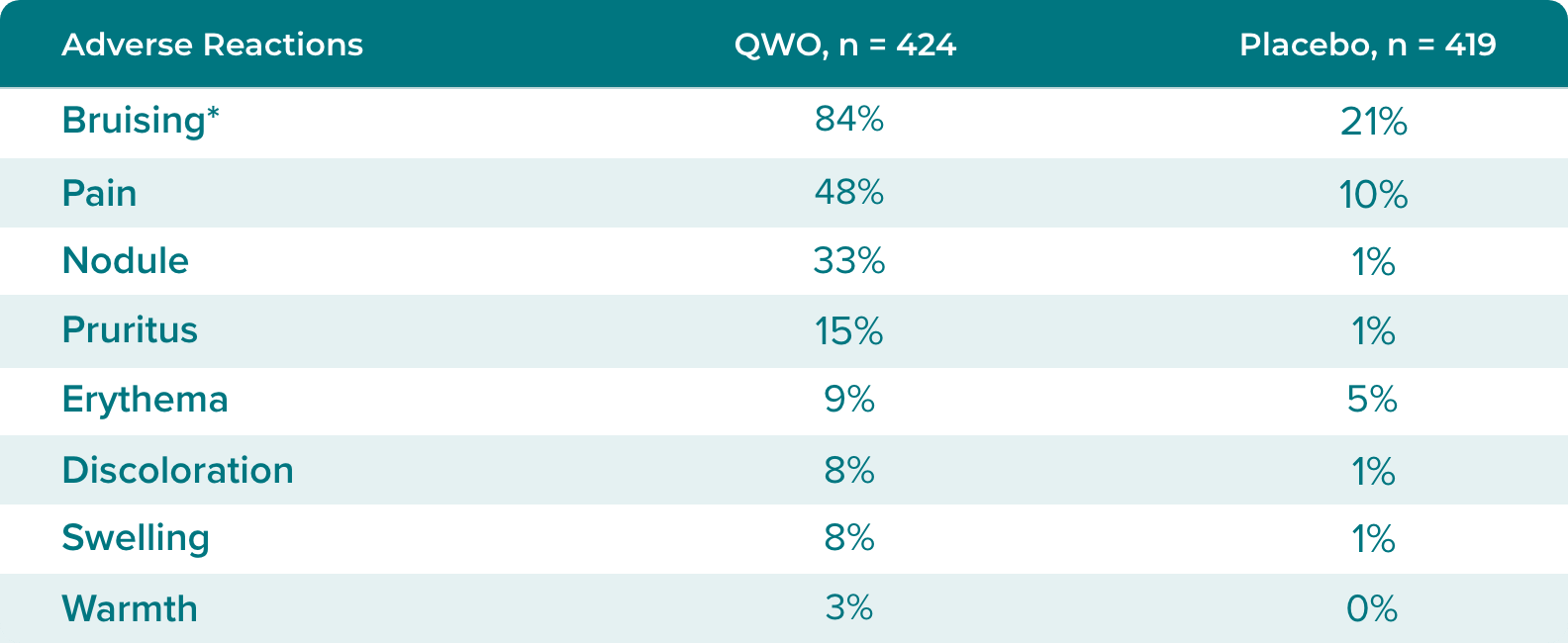
Bruising should be expected, as 84% of patients in clinical trials experienced bruising3
Adverse reactions occurring in ≥ 1% of subjects in RELEASE-1 and -2 through Day 713
Pooled Terms
*Bruising - injection site bruising, injection site hematoma, and injection site hemorrhage (refers to verbatim term injection site ecchymosis). Pain - injection site pain, injection site discomfort, and injection site dysesthesia. Swelling - injection site swelling, injection site edema, injection site induration. Discoloration - injection site discoloration. Nodule - injection site mass and injection site nodule.

Injection site bruising
In clinical trials, 84% of subjects treated with QWO experienced injection site bruising. Subjects with coagulation disorders or using anticoagulant or antiplatelet medications (except those taking ≤150 mg aspirin daily) were excluded from participating in Trials 1 and 2.
QWO should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet (except those taking ≤150 mg aspirin daily) or anticoagulant therapy.
Adverse events
No additional safety concerns were identified with six months of follow-up.3 In general, adverse events associated with QWO injection were mild to moderate and resolved within 2-3 weeks without treatment.1,3,10
Learn more about the safety of QWO.
Want to learn more about QWO?
Head over to the Clinical Education Center for more information.