Treatment
To help achieve desired results, patients should complete all three treatment sessions3


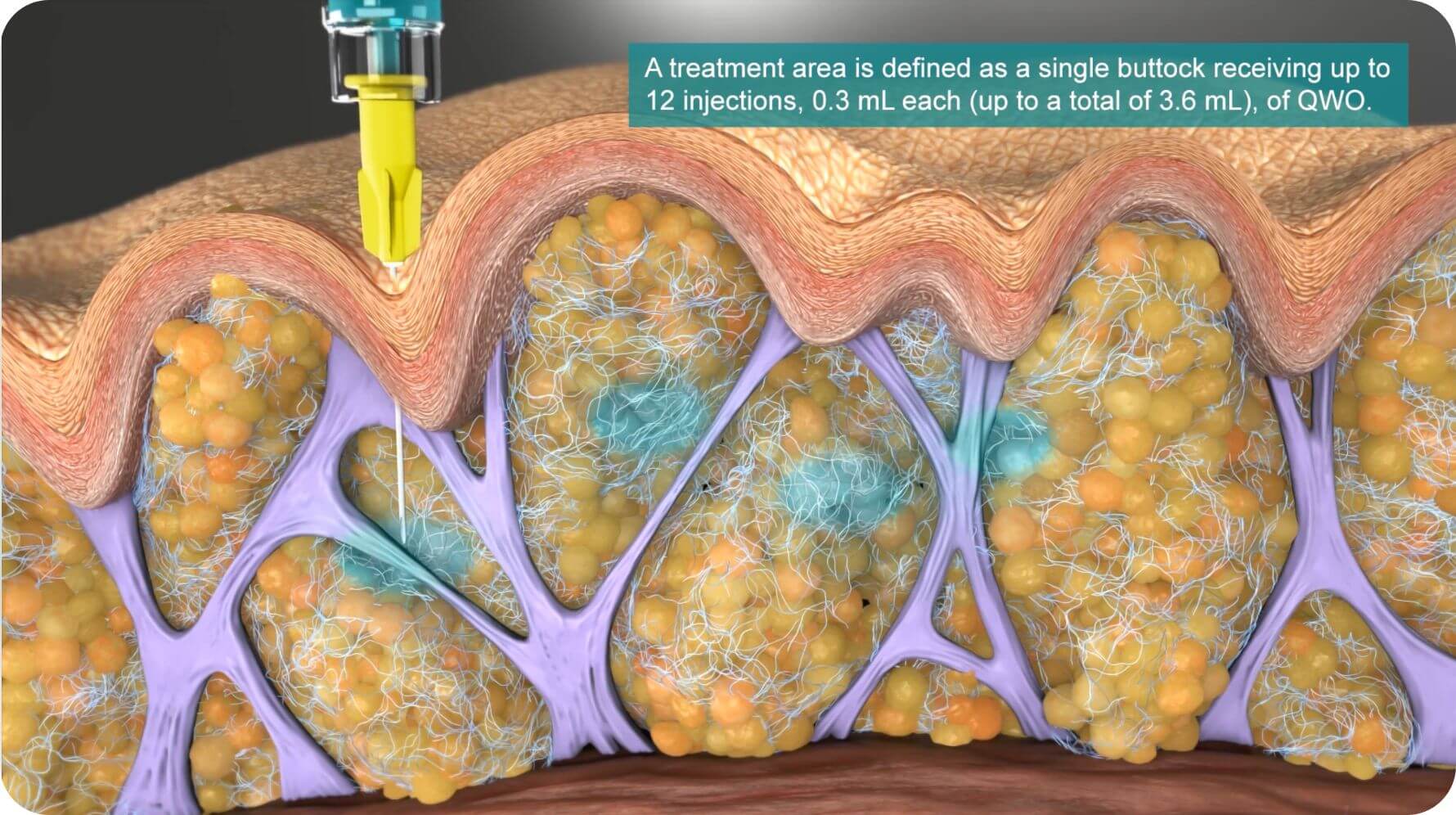
Each treatment
Patients may receive up to 12 injections per buttock, for a maximum of 24 injections for both buttocks.3
Some dimples may require more than one injection.1,10


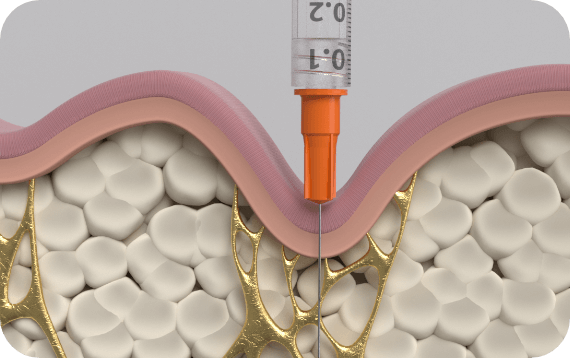
QWO injection technique:3
This injection technique is for the buttocks only.
- Use a 1 mL syringe with a 30 g 1/2” needle.
- Do NOT remove the needle from the injection site during the three-part injection – gently pull the needle back and reposition it.

Three-part injection technique:3
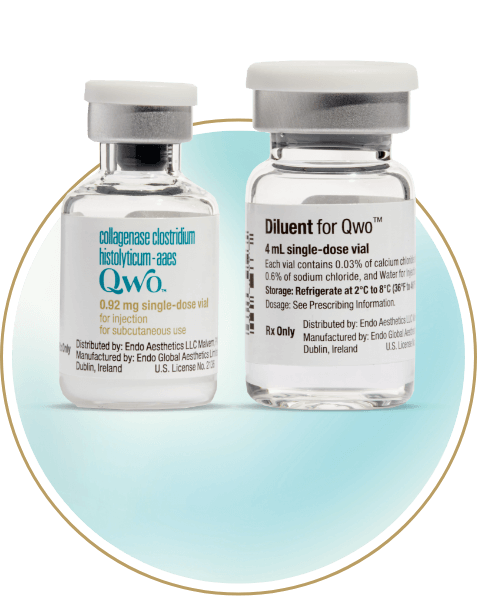
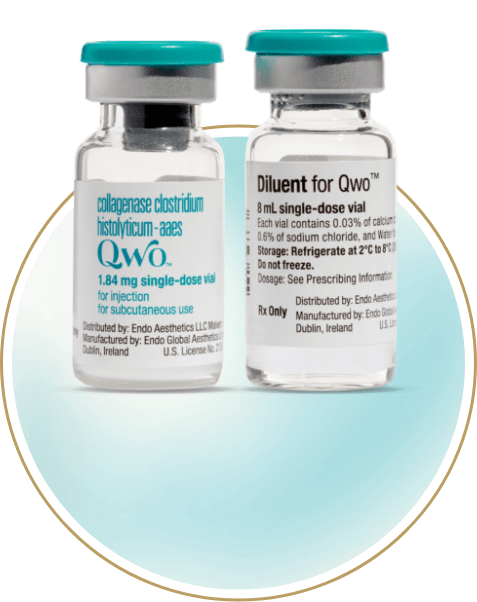
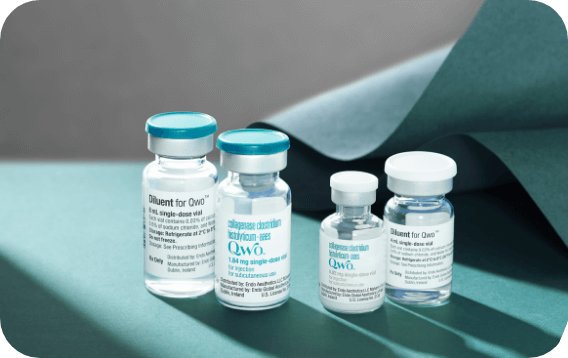
QWO is supplied as a sterile, preservative-free lyophilized powder in a single-use vial that must be reconstituted before use.3
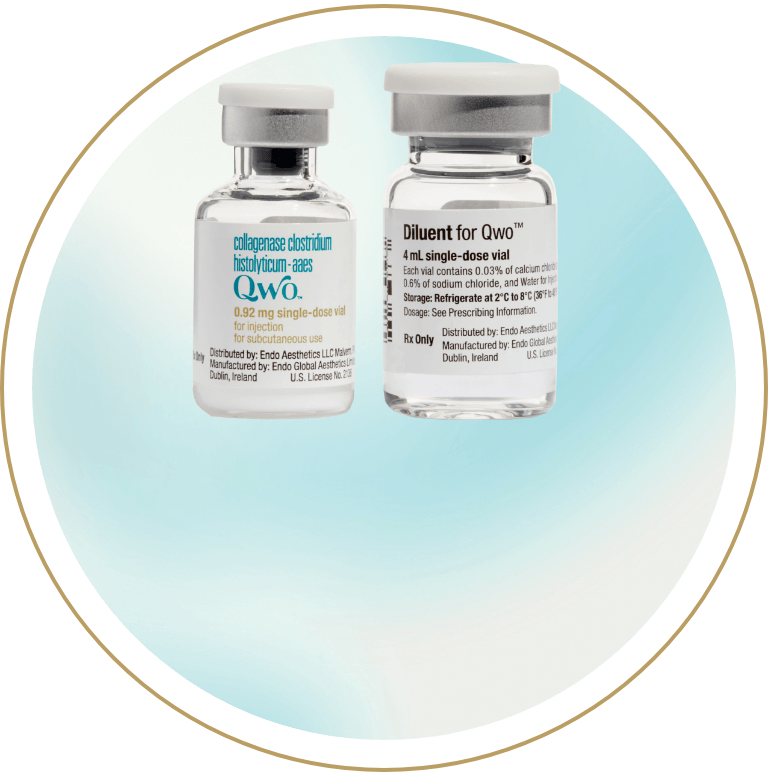
4 mL diluent
Up to 12 injections*
or
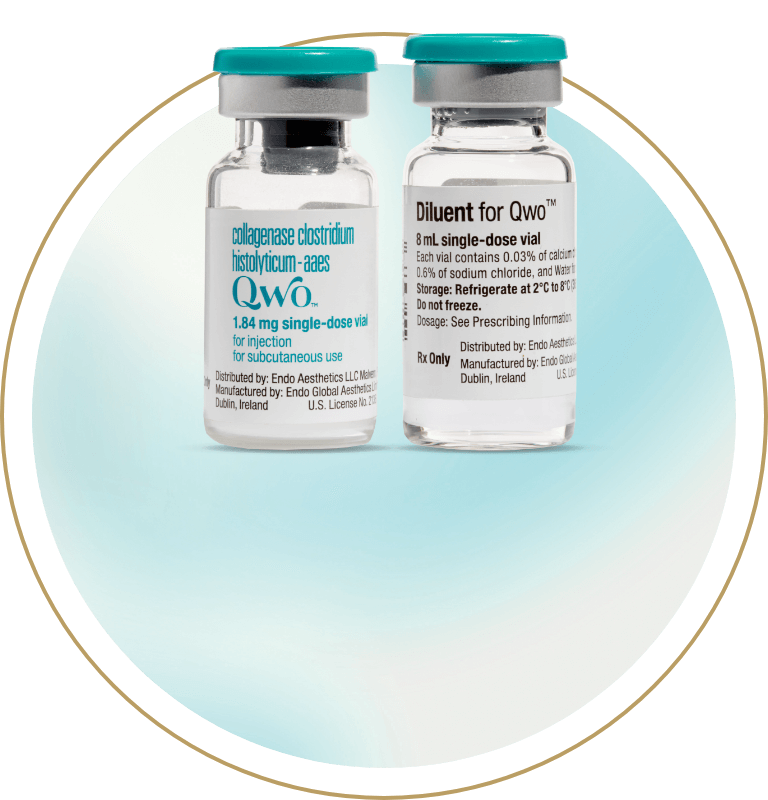
8 mL diluent
Up to 24 injections*
*There is a maximum of 12 injections per buttock, per treatment.
Clinical Education Center
Here you will find additional information and resources.
Carousel items
-

-

-

Publications & presentations
Read up on a variety of publications
Learn More
and presentations on QWO -